Ingredients and supplement facts can be found on each individual product page. To find AdvoCare® products and particular ingredients, enter the product name you wish to find into the search engine found on the top right of the menu on AdvoCare.com.
Please refer to the AdvoCare® Product Age, Pregnancy and Nursing Indications document to see guidelines for product appropriateness. These guidelines are not intended as a substitute for the recommendation of your healthcare professional.
AdvoCare® policy prohibits marketing or sales to individuals under the age of 18. We strongly recommend that decisions concerning any products that may be given to minors include parental and healthcare provider involvement.
If you are pregnant or nursing, please consult your physician to decide if an AdvoCare product regimen is appropriate for your needs.
Click here for the suggested Pregnancy and Nursing Indications.
These are general guidelines for pregnancy and nursing product appropriateness. They are not intended as a substitute for the recommendation of your health care professional. Consult your physician with any questions.
Many people have had success with the AdvoCare 24-Day JumpStart®. However, there are some products in the system that may not be suitable for every individual. This is why it is very important to check with your healthcare professional before beginning any regimen.
An alternative to 24-Day JumpStart® could include beginning with ProBiotic Restore™ Ultra, and then sequentially adding AdvoCare Fiber, an MNS® system, Catalyst™, OmegaPlex®, ThermoPlus®, Carb-Ease® Plus and Spark® as needed.
For more information, visit the 24-Day JumpStart® page
We recommend discontinuing all thermogenic products (MNS®️, ThermoPlus® and AdvoCare® PRE Workout) when using the AdvoCare 10-Day Reset™️. All other AdvoCare®️ products may be continued but should be taken several hours after consuming AdvoCare®️ Fiber so that optimal intestinal absorption of nutrients can occur.
The Metabolic Nutrition System (MNS®) is a packaged unit of multinutrient dietary supplements originally named Metabolic Nutrition System. Over time AdvoCare® has shortened the product name to MNS. Both MNS systems provide core nutrition, energy and metabolic support.* It is designed for anyone looking for additional support for appetite control, energy and overall wellness.
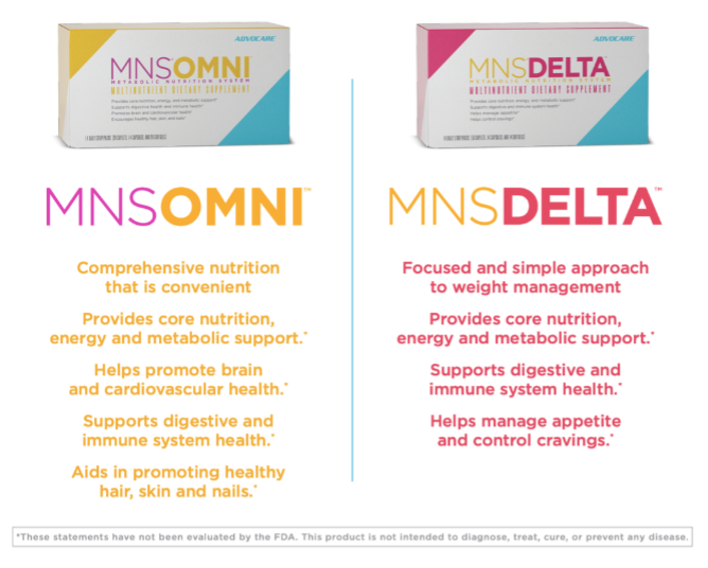
MNS Omni™ and MNS Delta™ can be taken in conjunction with other AdvoCare® products. These products contain botanical ingredients that have an equivalent to caffeine. We encourage users to monitor their caffeine intake while taking MNS.
If consuming fiber in conjunction with MNS Omni or Delta, it is important to consume AdvoCare Fiber at least 2 hours prior or 2 hours after taking OmniBlend or the Multivitamin Softgel to ensure optimal absorption of the nutrients. Fiber can reduce the absorption of oil containing products if taken around the same time.
For more information, visit the MNS page.
A mistaken belief still exists stating the dietary supplement industry is unregulated. A dietary supplement manufacturer is responsible by law for ensuring a supplement is safe before it is marketed. Manufacturers must also ensure product label information is truthful and not misleading.
The Food and Drug Administration (FDA), as part of the Department of Health and Human Services, and the Federal Trade Commission (FTC) share oversight of foods and dietary supplement manufacturers. Products themselves are addressed in a variety of regulations published in the US Code of Federal Regulations.
FDA regulates safety, quality systems, manufacturing processes, recalls and mandatory adverse event reporting. Product related information such as labeling, claims, package inserts and accompanying literature for foods and dietary supplements are also specifically addressed in regulations. These regulations are codified in the US Code of Federal Regulations in a variety of sections relating to Good Manufacturing Practices, labeling, product claims and ingredients.
FTC has primary responsibility for regulating advertising of dietary supplements in forms of media including social media and blogs. Advertisements and personal testimonies for dietary supplements must not make claims without substantiation by scientific evidence and must not be intended to diagnose, treat, cure, or prevent any disease. With the mutual goal of consumer protection, FDA and FTC, in conjunction, ensure combined jurisdiction over these products. Federal agencies in the U.S., Canada and Mexico also coordinate activities with regulatory counterparts around the world.
AdvoCare® product ingredients are selected based on their quality, bioavailability and purity from reputable suppliers that meet AdvoCare’s quality standards. These ingredients used in our products are tested for purity and potency at our contracted manufacturers prior to use. Where possible, botanical extracts are standardized for known active markers to deliver consistent results. The particular forms of vitamins, minerals, amino acids and other nutrients are chosen based upon their bioavailability.
AdvoCare® products are produced in facilities that adhere to Federal cGMP (current Good Manufacturing Practice) guidelines. Quality control and quality assurance programs are part of standard operating procedures at each facility. Caplets, capsules, softgels, drinks, shakes and bars are all made in accordance with very narrow quality control and product specifications. As result of these specifications and control procedures, consistently high quality products batches are released every time.
AdvoCare® bases its formulations on credible scientific research and sound nutritional principles. The Company utilizes established scientific knowledge as the foundation for our formulas while incorporating current research results from scholarly studies conducted around the world. AdvoCare® does not utilize ingredients based on passing popularity. Each formula is designed to be safe and effective when used as directed for its intended purpose.
AdvoCare® has formed a strategic partnership with Informed Choice and Informed Sport to certify the products that carry the logos are banned substance free. AdvoCare® values its relationships with the athletic community. With testing on the rise for performance-enhancing substances, both at the amateur and professional levels, this partnership allows athletes to make informed choices about what they put into their bodies.
Click here for more information.
The United States Pharmacopeia (USP) is a nongovernmental organization whose mission is to promote public health and benefit practitioners and patients. The core USP initiative is disseminating authoritative, state-of-the-art standards to ensure the quality and consistency of prescription and non-prescription drugs, dietary supplements, veterinary drugs and other healthcare products.
USP criteria for disintegration requires that tablets disintegrate within the prescribed time when placed in a liquid medium at the experimental conditions presented. Simply placing tablets in a glass of water and observing disintegration is not a legitimate test, and does not correspond to disintegration of tablets in the stomach. Meeting USP criteria for disintegration is a part of the standard manufacturing requirements and procedures for AdvoCare® products. For more information, please refer to https://www.usp.org/
Those with difficulty swallowing may chew or crush most AdvoCare® caplets. Exceptions include ActoTherm SR, and CraveCheck SR™. Since these caplets are formulated using sustained-release technology, chewing or crushing the caplet interferes with the extended-release mechanism and results in more rapid release of the ingredients. Capsules may be opened and sprinkled on food. The same procedure may be utilized for softgels, although the OmniBlend softgel ingredients may stain surfaces, skin and clothes.
The absorption rate of various nutrients is dependent upon a number of factors, including: individual metabolism; whether the nutrients are taken with food or on an empty stomach; types of foods ingested; supplement disintegration time; amount of physical activity; medical conditions present; medications taken; time of day the nutrients are consumed; form of the nutrient-how many steps are required for it to be broken down to the form that can be used by the body before absorption can occur; or physical form of the product (tablet, caplet, softgel, solution, suspension).
Unlike prescription drugs and over-the-counter (OTC) medications, the U.S. Food and Drug Administration (FDA) doesn’t require vitamin and dietary supplement manufacturers to include an expiration date on the packaging. Companies are responsible for making sure their products are safe.
FDA regulations require that companies utilizing best by dates to have product stability test data to support the shelf life. The agency considers determining a product's shelf life to be part of the manufacturer's responsibility.
Although expired vitamins may not be unsafe, they aren’t as effective as they once were. Most companies, including AdvoCare, have elected to display a “best by” on our product labels to indicate how long it is expected to last unopened before ingredient potency falls below 100 percent of the labeled amount. Another factor in deciding to include Best By dates is to inform consumers of the date up to which they can expect a product to retain desired quality and flavor.
The potency of many food and supplement products gradually decreases over time beginning on the date of manufacture. This degradation process speeds somewhat after packaging has been opened and may accelerate further if the product is exposed to heat, light and/or moisture. Instead of becoming unsafe to ingest, they become less and less potent.
Products should be stored in their original containers in a cool, dry place. If the products have changed markedly in color, consistency, texture or odor dispose of and replace them immediately. For optimum taste and product performance, it is always best to use products before the “Best By” date passes.
If your purchase does not meet your expectations for any reason, you may exchange your items or we will fully refund your money within 30 days of your purchase. Click to view details of the Satisfaction Guarantee.
AdvoCare® has a total satisfaction guarantee. We believe in our products. If they did not meet your expectations for any reason, call our Customer Service team at 1-800-542-4800 within 30 days of purchase. We will make it right. This is our promise.